
The 21st ATP of the CLP regulation introduces 28 new entries in the list of dangerous substances and revises the classification of another 24
On January 5, 2024, Commission Delegated Regulation (EU) 2024/197, of October 19, 2023, amending Regulation (EC) No. 1272/ was published in the Official Journal of the European Union. 2008, on the cla...
Read more
IMDG Code amendment 41-22 comes into force: overview
The maritime industry is witnessing significant changes with the introduction of Amendment 41-22 to the International Maritime Dangerous Goods (IMDG) Code. These amendments, already mandatory from Jan...
Read more
Understanding the Recent Amendments to KKDIK
On December 23, 2023, a significant shift in Turkey's chemical regulatory landscape unfolded as the Ministry of Environment, Urbanization, and Climate Change introduced a series of amendments to the "...
Read more
Canada launches new legislation to enhance dangerous goods transportation oversight
Last November 3, 2023, the Minister of Transport, Pablo Rodriguez, unveiled an groundbreaking initiative aimed at bolstering public safety and security concerning the transportation of dangerous goods...
Read more
SIAM USA LLC: Leading the Way in Corporate Social Responsibility
In today's dynamic business landscape, corporate social responsibility (CSR) has become more than just a buzzword; it's a crucial aspect of a company's identity and impact on society.
One stando...
Read more
CANADA - Updated WHMIS Guidance
Health Canada's Workplace Hazardous Materials Bureau has recently released comprehensive and updated guidelines pertaining to the supplier requirements within the Workplace Hazardous Materials Informa...
Read more
Brazil Implements Revision 7 of GHS: What is new?
In a move towards standardizing the classification, labeling, and safety documentation of chemical products, Brazil has adopted the 7th revised edition of the United Nations' Globally Harmonized Syste...
Read more
Dutch Flavor Ban on E-Cigarettes Set to Take Effect on January 1, 2024
In a significant development for the vaping industry in the Netherlands, the anticipated ban on flavored e-cigarettes is finally set to come into force on January 1st, 2024. This decision comes after ...
Read moreGet a better understanding of toxic chemicals, Proposition 65 warnings, and their impact on your health
Introduction:
This article aims to provide valuable insights to safety professionals in the chemical industry regarding toxic chemicals, Proposition 65 warnings, and their implications for heal...
Read more
EU Implements groundbreaking legislation to curb microplastics
In a move aimed at curbing plastic pollution, the European Union (EU) has rolled out new legislation, Commission Regulation (EU) 2023/2055, to regulate synthetic polymer microparticles, commonly known...
Read more
Getting ready for the upcoming Poison Centre deadline for industrial use only mixtures
Time has come for importers and downstream users of industrial use only mixtures to update their poison center notifications to fully comply with the harmonized information requirements outlined in An...
Read more
Addressing Formaldehyde Risks: EU Commission Introduces Stricter Restrictions for Consumer Articles
The European Commission has taken a significant step in safeguarding public health and environmental well-being by adopting a new regulation.
On July 14, 2023, the Commission approved Regulation (EU)...
Read more
ECHA's Workshop Paves the Way for Animal Testing-Free Chemicals Regulation
•Over 500 participants engage in the European Chemicals Agency (ECHA) workshop about New Approach Methodologies (NAMs) as alternatives to animal testing in chemical hazard assessment.
•The work...
Read more
Canada one step closer to ban “forever chemicals”
Although the Canadian government prohibited the manufacture, use, sale and import of three subgroups of per- and polyfluoroalkyl substances (PFAS1) (PFOS, PFOA and long-chain PFCAs) in 2012, the lates...
Read more
Canada updates its Hazardous Products Regulations: What's next?
The Canadian Ministry of Health published on January 4, 2023 in the Canada Gazette, the nation's official newspaper, the amendments to its Hazardous Products Regulation - HPR, with the aim of aligning...
Read more
When to update or revise your safety data sheets (SDS): a global guideUnderstanding regulatory requirements in countries that have adopted GHS
Understanding regulatory requirements in countries that have adopted GHS
Introduction
•Chemical suppliers must periodically review, and update their SDSs to incorporate new information about haz...
Read more
IT'S OFFICIAL: NEW CLP HAZARD CLASSES OFFICIALLY PUBLISHED
On March 31st, 2023, the European Union's Classification, Labelling and Packaging Regulation (CLP) underwent a significant amendment with the issuance of the delegated regulation EU 2023/707.
Th...
Read more
New UK Packaging Regulations. How will it affect my company?
Extended Producer Responsibility (ERP) is a principle that establishes that producers are responsible for the treatment of their products at the end of their useful life and for the waste they generat...
Read more
Entry 74: Diisocyanates restricted under REACH
The European Union (EU) has recently introduced a new restriction under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation for diisocyanates, which will come i...
Read more
Vietnam updates its Law on Chemicals
The Decree 82/2022/ND-CP, which came into force last December 2022, updates the existing Decree 113/2017/ND-CP and introduces several amendments to guide the implementation of the Law on Chemicals. Th...
Read more
EU vs UK CLP: What’s different and what can we expect?
When Brexit happened 3 years ago, it affected the chemical regulation relationships between UK and the rest of Europe.
Thus, the CLP Regulation was transposed into UK law after Brexit. Important...
Read more
The European Commission shares a draft for new hazard categories under the CLP
The objectives of the CLP Regulation are to ensure a high level of protection of human health and the environment, and the free movement of substances, mixtures and certain articles.
These objecti...
Read more
Do you need help to comply with the new Japanese SDS requirements? ¡New practical guideline published!
The Japan Chemical Industry Association (JCIA) has published a guidance to help companies to meet new SDS and labeling requirements under the revised ISHL.
Although it's not an official document, it ...
Read more
The European Commission consults the introduction of new hazard classes into CLP
Among the objectives of the CLP Regulation are (i) to ensure a high level of protection of human health and the environment and (ii) the free movement of substances, mixtures and certain articles.
...
Read more
Update of the ATEX regulations
In order to provide clarity, the European Commission has this time decided to publish in a single act/document the new list of harmonized measures on devices and protection systems in potentially expl...
Read more
Time is over: the new SDS requirements under Annex II will be mandatory for the next year
Regulation (EU) 2020/878, which modifies Annex II of the REACH Regulation and establishes new requirements for the safety data sheets of chemical products in the European Union, has been in effect for...
Read more
New labeling requirements of waste-generating products in France
The decree No. 2022-748 introduces a new consumer information system on the environmental impact of products during the act of purchase.
In 2020, France approved its "Anti-Waste and Circular Econo...
Read more
SVHC Candidate List extended to 224 substances
The list has recently added a new entry, N-(hydroxymethyl) acrylamide, due to its carcinogenic and mutagenic properties.
The Candidate List of Substances of Very High Concern - SVHC contains a lis...
Read more
NEW EUROPEAN DEFINITION OF NANOMATERIALS
The EU Commission clarify the definition of nanomaterials in the new Commission Recommendation of 10.06.2022 to support a “coherent EU regulatory framework for nanomaterials”.
And the fact is that...
Read more
Glyphosate, Yes: not change proposed to hazard classification
The latest RAC´s opinion recommends keeping the existing classification for glyphosate as a substance that causes serious eye damage and is toxic to aquatic life with long-lasting effects.
Just a y...
Read more
Singapore adapts to the GHS 7th Revised Edition
On April 4, the government agency Enterprise Singapore, which promotes the business development of the Asian country, published a revision proposal for the Singapore Standard (SS) 586, which regulate...
Read more
18th ATP of the CLP published
The Delegated Regulation (EU) 2022/692 was published in the Official Journal of the European Union on 3 May 2022 and will be applied from 23 November 2023.
The latest amendment to del CLP Regulatio...
Read more
Get ready for the new REACH information requirements
The ECHA has announced upcoming changes to certain REACH information requirements that will start applying from 14 October, 2022
The update of the REACH annexes, published in the Commission Regul...
Read more
34 bisphenols to be restricted due to their potential hormonal or reprotoxic effects
The ECHA´s Group Assessment of Bisphenols studied 148 compounds and recommended more than 30 bisphenols to be restricted due to their potential damage to human health and the environment.
The Grou...
Read more
Grace period in Korea: How to comply with the new MSDS requirements.
On November 2020, Korea's Ministry of Employment and Labor (MoEL) published major amendments to GHS Classification and MSDS requirements under the Notice No. 2020-130, which contains a major revision ...
Read more
At SIAM we are committed with the environment.
SIAM has recently obtained the certification of its environmental management system according to the international standard ISO 14001:2015, which demonstrates the company's commitment to preserve th...
Read more
Candidate List Updated with 4 New Hazardous Chemicals
The Candidate List of Substances of Very High Concern (SVHCs) now contains 223 entries identifying chemicals that are potentially harmful to human health and the environment.
You can consult the c...
Read more
Disabling a submitted notification: why, when and how
On December 2021, the ECHA updated the “PCN: a practical guide” to its version 5.0.
A version which includes a step-by-step explanation of the new functionality for disable submitted notifications th...
Read more
HOW CAN CHEMETER HELPS YOU TO COMPLY WITH UK´S PPE NEW REGULATIONS?
The UK´s Personal Protective Equipment at Work Regulation 1992 requires the employers to provide their employees with Personal Protective Equipment (PPE) to protect them from the risk of injury in the...
Read more
Most of the European products sold online do not comply with the regulations on chemical products
The latest report of the REF-8 project, which assesses compliance with the European regulations REACH, CLP and BPR, concludes that almost 80% of the online sales products inspected violated the legisl...
Read more
Following REACH registration and to eliminate SHVCs from plastic waste streams, keys to achieving non-toxic recycling
A new study by the ECHA focuses on the European Union situation and developments in chemical recycling.
Chemical recycling, a process by which plastic polymers are chemically broken down to supply...
Read more
#UFIMattersEU: ECHA´S new social media campaign on the importance of the UFI code
Last year, poison centers across Europe handled around 600,000 calls about accidental poisoning, and almost half of them reported incidents involving young children.
And is that, not being really awa...
Read more
SIAM ACQUIRES 100% OF MURCIAN COMPANY "LABORATORIOS ALMABE"
As part of SIAM's strategic growth and diversification plan, we have acquired the ownership of "Laboratorios Almabe IDUQC", an independent laboratory founded in 2012 and located in the Murcia Science ...
Read more
Echa´s new guideline to avoid animal testing and to reduce allergies caused by chemicals
The European Chemicals Agency has been working in collaboration with de OECD, the European Commission's Joint Research Centre and other organisations from the United States and Canada to develop alter...
Read more
PFCAs banned from 23 february onwards
The European Commission has published REGULATION (EU) 2021/1297 of 4 August 2021 amending Annex XVII to REACH restricting perfluorocarboxylic acids (PFCAs) containing 9 to 14 carbon atoms in the chai...
Read more
Doubts about the classification and labelling of titanium dioxide (TiO2) following regulation (EU) 2020/2017? New HELP GUIDE published!
The classification and labelling requirements for titanium dioxide (TiO2) changed in February 2020 following Delegated Regulation (EU) 2020/2017, effective October 1, 2021. To help companies comply wi...
Read more
SIAM collaborates with Vicente Ferrer Foundation in its "Oxygen for India" campaign
Since 1969, the Vicente Ferrer Foundation has been committed to the fight against poverty and the development of one of the most impoverished areas of India, the states of Andhra Pradesh and Telangana...
Read more
Colombia: new regulations to apply the GHS in the workplace.
The new Resolution 773 of 2021 of the Ministry of Labor defines the actions that employers must implement in their companies in terms of classification and hazard communication for chemical hazards, ...
Read more
8 new additions to the Candidate List
Last month, the European Chemicals Agency (ECHA) added 8 new hazardous chemicals to the Candidate List of Substances of Very High Concern (SVHC), which now contains a total of 219 chemicals.
S...
Read more
Enforcement Forum-38 meeting: main conclusions
The Forum will prepare a draft revision of the SDS in 2022, carry out inspections in 2023 and report the results in 2024.
The 38th meeting of the Enforcement Forum once again ratified the poor qual...
Read more
Glyphosate yes or no: AGG proposal does not contemplate changing the existing classification
The use of the most widely used herbicide in the world is in question after a WHO report warning of its "possible carcinogenic effects".. In Europe its use is guaranteed until 2022, but EFSA and ECHA ...
Read more
NO SAFE! Titanium dioxide E171 no longer safe when used as food additive.
An expert panel of the European Food Safety Authority (EFSA) has concluded that they cannot exclude genotoxicity concerns after consumption of titanium dioxide particles.
Titanium dioxide is used as ...
Read more
Update of the guidance on poison centres notifications to version 4.0.: main novelties
The Guidance on Poison Centres Notifications is an orientation document on the European harmonised information relating to emergency health response – Annex VIII to CLP, that aims to help users subje...
Read more
Published the 16th ATP of the CLP regulation
On April 20 it was published in the Official Journal of the European Union, the COMMISSION DELEGATED REGULATION (EU) 2021/643 of 3 February 2021, which is equivalent to the 16th adaptation to technica...
Read more
Member States decisions in relation to implementation of Annex VIII to CLP Regulation: updated document
Annex VIII harmonized poison centre notifications (PCN) was first implemented from January 1, 2021, for consumer and professional users. Importers, downstream users and distributors placing hazardou...
Read more
Canada proposes to align its hazardous products regulation with GHS, 7th revised edition
Adopting the seventh revised edition of the GHS would provide increased worker health and safety benefits and worker protections and to comply with the Canada – United States Regulatory Cooperation Co...
Read more
SVHC Roadmap 2020: a comprehensive analysis of its achievements
The roadmap, set up to identify and include all relevant Substances of Very High Concern (SVHCs) in the Candidate List by 2020, has just published an analysis of its main achievements and extended aim...
Read more
The Hazard Communication Standard (HCS or HAZCom) aligns with the Globally Harmonized System of Classification and Labeling of Chemical Substances (GHS) (revision 7)
New standard to align the HCS with the GHS
US Occupational Safety and Health Administration (OSHA) issues a proposed rule to update the current standard
The Occupational Safety and Health Administra...
Read more
New EU safety data sheet requirements: Transition period confirmed
SDS compiled in accordance with Regulation (EC) No 1907/2006 can continue to be used until 31 December 2022
The European Chemicals Agency ECHA published last December 2020 the fourth version of its...
Read more
Second Amendment of Annex VIII published to facilitate compliance
On November 13, the Second Amendment of Annex VIII to CLP was published in the Official Journal of the European Union. It was designed to improve the viability of the information requirements related ...
Read more
Problems at Customs due to the new Mexican NOMs? SIAM have the answer
At Siam we specialize in helping companies comply with all international regulations, so whether you have retained merchandise at Mexican customs or if you are thinking of exporting there, we can help...
Read more
Published the 15th ATP of the CLP Regulation - application of the regulation (UE) nº 2020/1182
The objectives of Regulation (EC) No 1272/2008 on classification, labelling and packaging of substances and mixtures (CLP) are to ensure a high level of protection of human health and the environment ...
Read more
NEW Requirements for EU Safety Data Sheets
The European Commission has published Regulation (EU) 2020/878, amending the annex II of REACH regulation, that sets the requirements for chemical safety data sheet in the European Union.
Regulation ...
Read more
THE EUROPEAN COMMISSION LAUNCHES AN INFORMATIVE DOCUMENT WITH RECOMMENDATIONS TO ADDRESS THE BREXIT IMMINENT ARRIVAL
The European Commission has just published "Readiness", an informative guide on preparations for the end of the transitional period between the European Union and the United Kingdom and which is addre...
Read more
Four new chemical substances included in the Candidate List of substances of very high concern (SVHCs)
The list, that now contains 209 substances that may have serious effects on our health or environment, has recently been updated to include three substances considered toxic to reproduction and one ...
Read more
SAFER CHEMICAL CONFERENCE: looking for a European chemical industry more environmentally friendly
How are we delivering on safer chemicals?
How can companies prepare for the new EU obligations on chemicals kicking in next year?
These are the questions that the European Chemicals Agency ECHA want...
Read more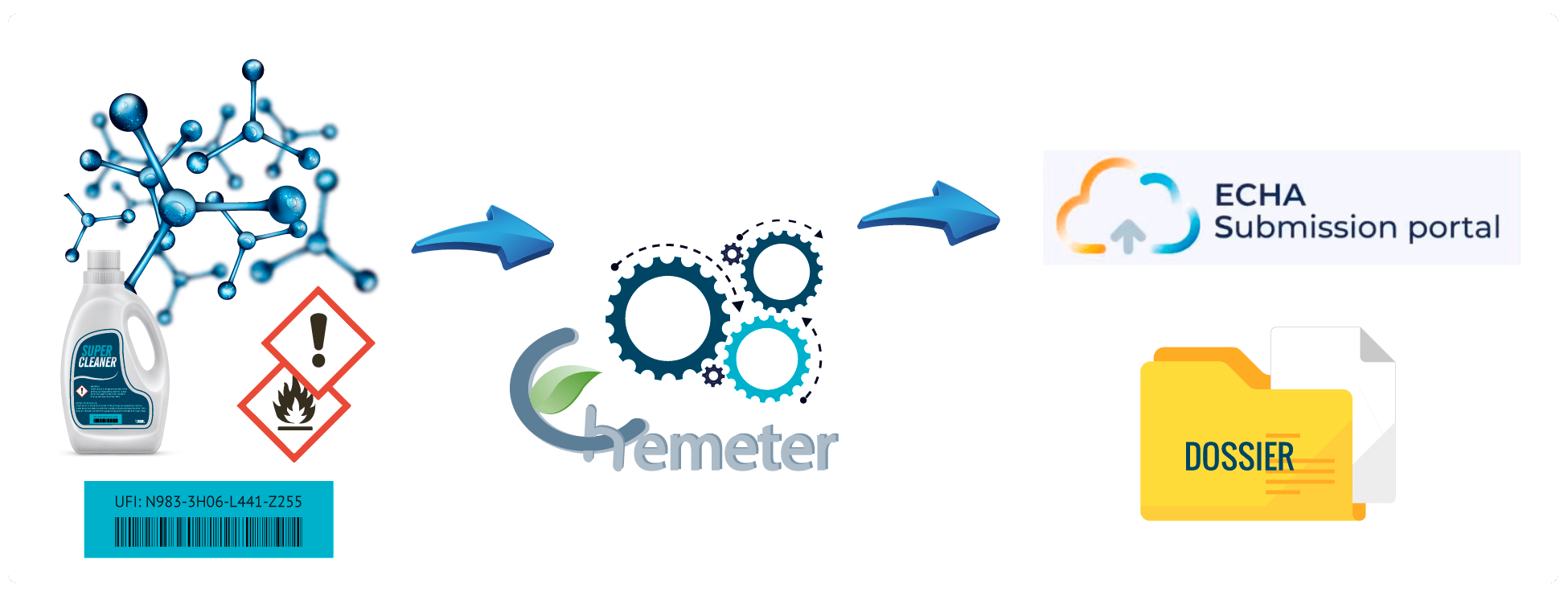
Siam participates in SURFEX LIVE 2020 with a webinar on European Harmonized Toxicological Notification
A few weeks ago, our teammates Pilar Ortiz-Alías and Nirav Banker were part of the SURFEX LIVE event with its webinar “Poison Centre Notifications (PCN): The UFI Code and European Harmonised Toxicolo...
Read more
800 250 250 becomes the only number of the INEM Poison Information Center (CIAV)
From next july, the old number will no longer be operating and 800 250 250 becomes the only number enabled for calls to the poison centre linked to the National Institute of Medical Emergencies (INEM)...
Read more
SIAM RELAUNCHES ITS CORPORATE STRATEGY IN LINKEDIN. DO YOU JOIN OUR NETWORK?
16 years after its birth, Linkedin has over 600 million users worldwide. Along this time, it has grown from a mere bulletin board and job portal to become a platform that gives voice to the organizati...
Read more
ECHA publishes on May its Guidance on harmonised information relating to emergency health response - Annex VII to CLP
A large number of chemical products (paints, sanitary products, detergents, etc) are placed on the EU market every year, and used by both the general public and professionals on a daily basis. Chemica...
Read more
Siam collaborates with Epson in its range of on-demand label printers
En Siam collaborates with Epson in its ColorWorks C6000 Series, and its models CWC6000P and CWC6000A, which allow to generate batches of color labels, on demand, and are equipped with a very intuitiv...
Read more
SIAM thanks all the professionals in the chemical industry
The entire SIAM team wants to thank the more than 3,000 companies and each of the more than 200,000 professionals in the Spanish chemical industry, who continue working day after day to fight the viru...
Read more
SIAM is committed and makes its contribution in the fight against COVID 19
SIAM has joined five other riojan companies to obtain 100,000 masks and 5,000 white coats, which have been donated to the Riojan Health Service.
...
Read moreFirst steps towards Eurasia REACH
updated new on 14/05/2020: Due to the COVID-19 pandemic, Russia’s ministry of industry and trade announced to extend the deadline for existing chemical inventory notification. The old deadline was bef...
Read more
EUCLEFT, an online search tool for EU chemical legislation
The European Chemicals Agency (ECHA) has just launched EUCLEFT, an online search tool for EU chemical legislation.
...
Read more
ECHA, FDA and other organism together against the COVID-19
NOTE: The information will be updated as soos as we get news in order to keep you up to date about this topic.
Against the global crisis that is causing the coronavirus disease (COVID-19), many gove...
Read more
The European Commission classifies titanium dioxide (TiO2) as a carcinogen by inhalation
On 4 October 2019, the European Commission adopted a legislative proposal to classify titanium dioxide (TiO2) as a carcinogen (carc.2), leaving 2 months to present the relevant objections.
...
Read more
ATP 14 - application of the regulation (UE) nº 2020/217
Regulation (EC) 2020/217, ATP 14 (Adaptation to Technical Progress) which amend, for the purposes of its adaptation to technical and scientific progress Regulation (EC) No 1272/2008 of the European Pa...
Read more
44% of the mixtures considered as hazardous do not comply with the mandatory classification and labelling!
(Legislation “breaks down” in the industry)
Inspectors from 29 countries revised a total of 3391 mixtures and 1620 companies from manufacturers, importers, downstream users and distributors in the si...
Read moreWebinar Poison Centre Notifications 2020 (ECHA)
Next 12th February from 11:00 to 13:00, the European Chemical Agency (ECHA) will offer an English webinar about the latest and upcoming news for the European harmonised notification (Annex VIII). ...
Read more
Update of the Candidate List of Substances of Very High Concern (SVHCs)
The Candidate List of substances of very high concern (SVHCs) has been upgraded to 205 substances. The ECHA (European Chemical Agency) has just included 4 new substances; due to their toxicity to re...
Read more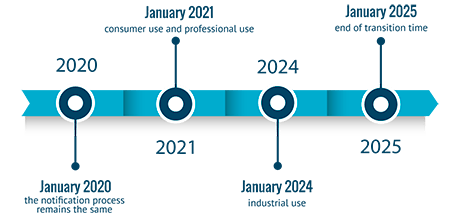
It is official already: The postponement to 2021 of the harmonised European format for notifications
Last 10th January 2020, was published the definitive text of the Regulation (EU) 2020/11 (shall apply from 1 January 2020) which amends the Regulation 1272/2008 (CLP), specifically the Annex VIII. Thi...
Read moreUFI code and harmonised toxicological notification – Current status
On 29th November the resolution of the last meeting held between the Competent Authorities for REACH and CLP (CARACAL) was published. The European commission agreed on amending the text for the CLP An...
Read moreGulf States to implement GHS in 2020
The trade bloc of the six Gulf Cooperation Council countries (Bahrain, Kuwait, Oman, Saudi Arabia, the United Arab Emirates and Qatar) expects to adopt the UN GHS (Globally Harmonized System) on class...
Read more
It is already available the eighth revised edition of the GHS
Although many countries are still updating and even implementing the seventh revised edition of the GHS, the Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized Sys...
Read morePU Consulting AB new SIAM’s partner
In SIAM we are committed to expansion and that our products reach as many customers as possible. That is why we are pleased to announce the collaboration with PU Consulting AB. This will allow custome...
Read more
ECHA prioritizes 18 substances (SVHC) on the Candidate List for Authorisation
ECHA has published its ninth recommendation to the European Commission regarding substances of very high concern (SVHC) for the REACH Authorisation List.
...
Read moreYou are still on time to give your opinion about changes in REACH, nanomaterials and poison center
The European Commission has launched a public consultation on an amended the European REACH Annex II. The main changes under discussion will affect the following topics:
...
Read more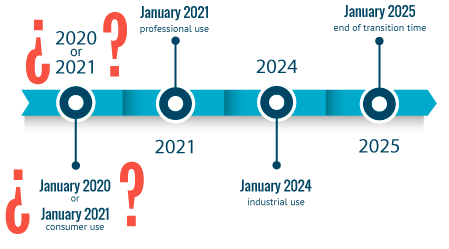
Public consultation on postponing the date of entry into force pf Annex VIII (UFI & harmomised notification)
The European Commission has launched a public consultation on an amended Annex VIII’s draft. The main update is the change of the compliance date to submit information on hazardous chemical mixtures f...
Read more
4 new substances added to the Candidate List of Substances of Very High Concern (SVHCs)
The Candidate List of substances of very high concern (SVHCs) has been upgraded to 201 substances. The ECHA (European Chemical Agency) has just included 4 new substances; due to their toxicity to re...
Read more
Replace "UFI notified to the Spanish INTCF" by the "harmonized UFI"
INTCF (Spanish Institute of Toxicology and Forensic Sciences), communicate to all the notifiers companies, the option to replace the previously notified ‘UFI’ of the mixture in Spain by the actual ha...
Read more
50% of the SDS verified contained errors
ECHA publishes the “Report on Improvement of Quality of SDS (safe data sheet)”.
...
Read moreCIAV, new emergency contact number
As of July 1, 2019, CIAV emergency contact number will be changed to a toll-free number – 800 250 250.
...
Read more
Main modifications on ADR 2019
Dangerous goods safety advisor for sender and comercial agents
Following the 1.6.1.44 point, it’s compulsory to designate the senders or comercial agents who only hire but not load, unload or transpo...
Read more
SIAM’s first time in the CPhI North America
From April 30th to May 2nd SIAM took part as an exhibitor in the CPhI North America, which was developed in Chicago.
...
Read more
Siam advises in Finland on the "trending topics" of chemical legislation
Pilar Ortiz, a member of Siam’s legislative support team, took part on the Seminar organized by Chementors, a chemical safety consultant and our partner in the Scandinavian country.
...
Read more
Published ATP 12
Regulation (EC) 2019/521 (ATP 14) which amend, for the purposes of its adaptation to technical and scientific progress Regulation (EC) No 1272/2008 of the European Parliament and of the Council on cla...
Read more
SIAM sponsors young motorcyclist Paul Dufour
The French motorcyclist runs this season for JEG Racing team, which is based in the Valencian circuit of Cheste.
...
Read more
SIAM at the european coatings leader exhibition
SIAM attended from 11 to 14 February in Nuremberg (Germany) to the European Coatigns Show, the leading coatings show.
...
Read more
Ready, steady… Brexit!
On March 13, the Business Federation of the Spanish Chemical Industry (FEIQUE) in collaboration with ICEX Spain Export and Investment, organised the event: "BREXIT and the implications of supply and u...
Read more
SIAM in Specialty & Custom Chemicals America
SIAM attended from February 11 to 14 in Fort Worth (Texas)) to the Chemical America fair through its company in the United States SIAM LLC.
...
Read more
Connected Regulations in the Rubber sector
Last 1st February was conducted an online seminar for members of the "Consorcio Nacional de Industriales del Caucho" (Spanish Rubber Association) about “Connected Regulations in the Rubber sector: ADR...
Read more
ATP10 – Application of the Regulation (UE) nº2017/776 to the CLP Regulation
Since 1st December the 10th Adaptation to Technical and scientific Progress (ATP) is in force and applicable to the Regulation nº1272/2008 (CLP). This new adaptation modifies the harmonised classifica...
Read more
6 new substances added to the Candidate List of Substances of Very High Concern (SVHCs)
The Candidate List of substances of very high concern (SVHCs) has been upgraded to 197 substances. The ECHA (European Chemical Agency) included last 15th January 6 new substances; 5 of them due to the...
Read moreHuge attendance of new seminar about NOM-018-STPS-2015 organized by EPSON & SIAM MEXICO
SIAM and EPSON scheduled again three sessions about NOM-018-STPS-2015 in México....
Read more
Success of the Conferences about the NOM-018-STPS-2015 organized by EPSON and SIAM MEXICO
During last September, the team formed by two reference companies in its field, SIAM and EPSON, held three days of dissemination on this occasion in Mexico about the NOM-018-STPS-2015.
The sessions...
Read moreNOM-018-STPS2015: it has finally become effective!
On october 9th the NOM-018-STPS-2015 came into effect compulsorily in México, which means to leave without any effect the previous NOM-018-STPS-2000.
...
Read moreThe kick-off of the ECHA to the revision project of Safety Data Sheets
The ECHA has formed a working group with the purpose of identifying trends and the greatest shortcoming of the Safety Data Sheets in order to set the follow-up actions for the year 2019.
...
Read morewe guarantee your data security
Good news! SIAM has implemented the new General Data Protection Regulation which is in force since May 25th.
...
Read more
We work with and for our clients
SIAM are committed with achieving high quality standards in all our processes and working procedures. Thanks to the work of our team, we have just been ISO 9001:2015 certified.
...
Read moreATP11 to the CLP Regulation
The 11th adaptation to technical and scientific progress (ATP) to the CLP Regulation had already been officially published by the EU Commission on 16 April.
...
Read moreThe use of methanol in windscreen washer fluids has been restricted
The use of methanol in windscreen washer fluids has been restricted by the Regulation (UE) 2018/589.
...
Read more
American Coatings Show 2018
Last April 10-12, the American Coatings Show took place at the Indiana Convention Center in Indianapolis.
...
Read more
Third consecutive assistance of SIAM at Turkcoat
Paintistanbul Turkcoat, one of the most important and globally consolidated trade shows took place last 22-24 March in Istanbul.
...
Read more
GLOBALCHEM 2018 – Conferences about global chemical regulations
SIAM USA LLC attended the GLOBALCHEM 2018, event gathering the worldwide cutting-edge chemical regulations experts. It took place in Washington from February 28th to March 2nd, being hold by the Ameri...
Read moreWhat is the UFI number?
Owing to the importance of this labeling element for products subjected to CLP classification, ECHA has published a video and information about it here.
...
Read more
ADELMA TECHNICAL COMMISSION ANNUAL MEETING on the 15th February, 2018
SIAM, as every year, held a presentation at the annual technical commission of the Spanish Association for Detergents and Cleaning and Maintenance products. SIAM focused this edition GHS adaptations o...
Read more
2017 SIAM-EPSON TOUR ENDS IN SEVILLE
After touring around the Iberian Peninsula (Barcelona, Madrid, Lisbon, Porto and Murcia) SIAM-EPSON conferences finalize in Seville on the 7th February, 2018.
Thank you for your participation and h...
Read more
CHEMCON THE AMERICAS 2018
The ChemCon Conferences event was hold in New Orleans from the 5th to the 9th of February, where worldwide chemistry experts were met.
...
Read moreSeven new substances added to the Candidate List
The Candidate List of substances of very high concern (SVHCs) for authorisation now contains 181 substances.
...
Read moreHow UK´s withdrawal from the EU impacts chemical products?
From 30 March 2019, UK will be no longer part of the EU and it will become a « third country » outside the Union.
...
Read moreTranslations of the Guidance on labelling and packaging according to CLP now available
Following the update of the Guidance on labelling and packaging (version 3.0) published in July 2017, the corresponding translations have now been published on the ECHA website and are available in 23...
Read more
Methanol restriction for windscreen washing or defrosting fluids and denaturated alcohol moving forwards towards restriction
REACH Committee of the European Commission, in the meeting held the 24th and 25th October, gave a positive opinion on the methanol restriction project.
...
Read moreSIAM participates in the 27th technical congress of ASEFAPI
ASEFAPI (Spanish association of Paint and printing inks manufacturers)
Once again SIAM took part at the technical congress of ASEFAPI at the Santiago Bernabeu stadium (venue).
...
Read moreThe new order about toxicological file in Spain is a reality
The Official Spanish Gazette published on the 29th of September 2017, the order JUS/909/2017....
Read moreARGENTINA STABLISHES GHS
Argentina has implemented the UN’s Globally Harmonized System (GHS) for the classification and labelling of chemicals.
...
Read moreNew Eurobarometer study on chemical safety
The new Eurobarometer study on chemical safety shows that 65 % of EU citizens are concerned (of 28 000 EU citizens interviewed) , to different extents, about being exposed to hazardous chemicals. Wh...
Read moreSouth Korea and Japan update GHS substance classification List
South Korea's National Institute of Environmental Research has added 23 substances and has revised 50.
...
Read moreThe 74th Florida Feed Association Convention
From July 13 to 15, 2017, the 74th Florida Feed Association Convention was held in Bonita Springs, Florida.
...
Read more
Latin America Coating Show 2017
For the first time since its creation early in 2017, SIAM USA had an outstanding performance at the Latin America Coating Show in Mexico, at the Banamex Convention Center on June.
...
Read more
RFME Spanish speed championship: Circuit of Navarra
During the weekend of the 24-25, of June took place the third round of the RFME Spanish speed championship at Circuito de Navarra, close to Logroño.
...
Read more
RUBBER CONNECTED 4.0
The national consortium of Rubber industries hold the XXIIth edition of its technical conference “Rubber Connected” on June 1st at ITAINNOVA (Technological institute of Aragón)
...
Read more
Seminar on “Segurança dos produtos químicos” in Portugal
More than 70 professionals registered for the SIAM-EPSON conferences in Lisbon and Porto.
...
Read moreLast call to remove from the market chemical products with old labels : 1st of June 2017
From 1 June 2017, all chemical products placed on the market have to be labelled in accordance with the CLP Regulation.
...
Read moreNew York is set to become the first US state to require cleaning product manufacturers to publicly disclose ingredients and to identify chemicals of concern used in formulations
The state is introducing the Household Cleansing Product Information Disclosure programme. The state's Department of Environmental Conservation (DEC) released a draft certification form and guidance d...
Read moreChemical products with old labels off the shelves
From 1 June 2017, all chemical products placed on the market have to be labelled in accordance with the Classification, Labelling and Packaging (CLP) Regulation.
...
Read more
Conference at EPSON Iberica headquarters
On April 27th, Siam and Epson Iberica organized a seminar where more than 75 people from chemical industry attended.
...
Read moreEU endorses restrictions to CMR substances
It has been approved the inclusion of 25 new substances to REACH appendices 1 to 6, resulting in a modification of points 28,29 and 30 of the Annex XVII.
...
Read more
SIAM USA and SIAM join forces!
During the last week of March, US SIAM team visited SIAM headquarters in Logroño.
...
Read more
25 years since the creation of EU ecolabel!
23rd of March was Ecolabel anniversary. It has been 25 years since the EU Ecolabel was launched and adopted at EU level and currently up to 40000 products and services are covered by this scheme.
...
Read more
SIAM successfully attended to the European Coating Show!
For the fourth consecutive time, Siam attended to the most important worldwide show of paints, vanishes sealants, building chemical and adhesives; the European Coating Show 2017.
...
Read moreSIAM has attended to tecnova Piscinas trade fair
Between 28 February and 3 March, IFEMA exhibition ground hosted the first edition of the Technology and Innovation for Swimming Installations trade fair, Tecnova Piscinas (http://www.ifema.es/tecnovap...
Read more
On March 24th, SIAM participated in the annual APT Seminar
On March 24th, SIAM introduced its CHEMETER and SDSArea solutions in the annual APT Seminar (Portuguese paint and coating association).
...
Read morePublished Regulation (EU) 2017/542
Published Regulation (EU) 2017/542 amending CLP regulation by adding Annex VIII on Poison centers harmonized information requirements
...
Read more
SIAM participated in the charity paddle sporting competition “Paladas por la donación”
Siam took part in the Paddle tournament supporting the organ donation held in Logroño from March 10th to March 12th 2017.
...
Read more
ADELMA technical commission annual meeting
On February 23, SIAM participated in the annual technical commission of ADELMA. Siam´s speaker talked about the Harmonization of toxic notifications at EU level in a nutshell.
...
Read more
New subsidiary in Miami, Siam more global than ever
The presence and fast expansion of SIAM through the five continents is a reality, so, in order to perform at our best and improve our service worldwide a new subsidiary has been opened in Miami. This ...
Read moreWatch out! The European Commission has published corrections to the CLP Regulation Nº 1272/2008
On December 21st 2016, The European Commission has published corrections to the CLP Regulation (EC) No 1272/2008 on classification, labelling and packaging of substances and mixtures, amending and rep...
Read moreDo you know the Forum for Exchange of Information on Enforcement (Forum)?
This body is a network of authorities responsible for the enforcement of the REACH, CLP and PIC regulations in the EU, Norway, Iceland and Liechtenstein.
This forum is of great interest for our clien...
Read moreNew siam website
Welcome to our new website! At SIAM we have always excelled in providing benefits tailored to each customer. That's why, with this new and improved image, we hope to make navigation more comfortable a...
Read more
SIAM partners with CESFAC
On the 28th of September 2016, SIAM played a key role in the meeting of the fourteen regional organisations which make up the Spanish Confederation of Animal Feed Manufacturers (CESFAC).
...
Read more
Supporting motorcyclist Paul Dufour in the National Speed Championship
The SIAM IT Company is truly committed to sports involving cutting-edge technology, dedication and teamwork, values which are reflected in its business philosophy. This is why the leader in software f...
Read more
SIAM at HPCI in Poland: a success for Chemeter
For the fifth year running, SIAM attended the Home and Personal Care Ingredients (HPCI) exhibition, which was held on the 28th and 29th of September 2016 in Warsaw.
...
Read more