
Echa´s new guideline to avoid animal testing and to reduce allergies caused by chemicals
The European Chemicals Agency has been working in collaboration with de OECD, the European Commission's Joint Research Centre and other organisations from the United States and Canada to develop alternative methods to assess chemical hazards and to protect people from skin sensitisation while prompting non animal test methods.
The result of this collaboration is the Guideline on Defined Approaches for Skin Sensinitation, adopted on June 14, 2021.
Since 2017, REACH requires registrants to use in vitro methods (tests outside a living organism, usually involving isolated tissues, organs or cells) to provide data on skin sensitisation. They also promote the use of in silico tools, which consist of computer simulations with programs such as the QSAR Toolbox developed by ECHA and the OECD.
From information in ECHA’s Classification and Labelling Inventory, there are over 14 000 substances on the EU market with some indication of a skin sensitising concern. Given the high number of substances, it is considered of great importance to evaluate the sensitizing effects in order to control (and restrict if necessary) their use.
Now, the ECHA has published a new guideline to advice registrants on how to reliably combine the different sources of alternative data provided by the OECD Defined Approaches for Skin Sensinitation.
What's in the new guide?
- 1. Annex VII to the REACH Regulation includes a requirement for in chemico/in vitro tests as a first step for addressing skin sensitisation (Section 8.3.1). The guide contains an overview of the available internationally validated in chemico/in vitro methods and they are presented in this table:
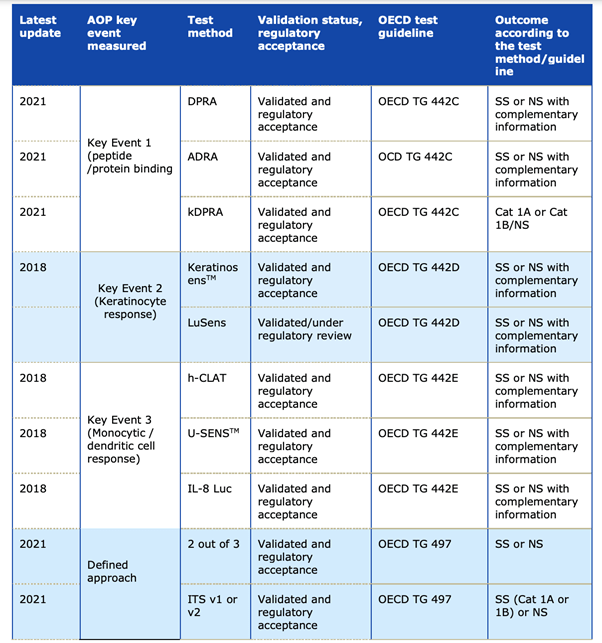
Source: Echa´s guideline on Skin Sensitization
- 2. It also includes a detailed explanation on how to use these non-animal test methods and helps the registrant to ensure which of these test methods are suitable for each substance to obtain adequate information.
- It explains how to apply defined approaches (DA) to testing and assessment, defined as a fixed data interpretation procedure (DIP) used to interpret data generated with a defined set of information sources, that can either be used alone or together with other information sources, to satisfy a specific regulatory need.
- 3. The guideline also helps registrants who have already submitted in chemico or in vitro data in their registrations but are uncertain whether this data is accepted by regulators. ECHA encourages those registrants to check whether they can use the defined approaches for their substances to make conclusive predictions and update their dossiers accordingly.

Let's talk!
Newsletter


